Development Safety Update Reports (DSURs)
Semi Annual SUSAR Reports
Risk Management Plans (RMPs)
(EU & Core)
(EU & Core)
Safety Modules of eCTD: Addendum To Clinical Overview (ACO)
Targeted Safety Evaluation
Established Product Safety & Efficiency
Regulatory Filing Strategy & Submission
Risk Management & Long-Term Safety
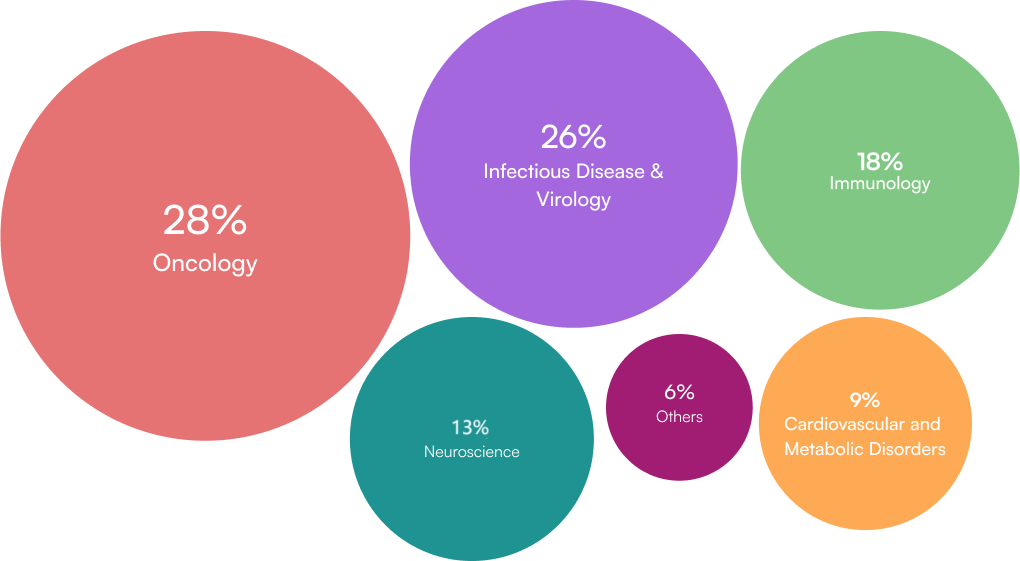
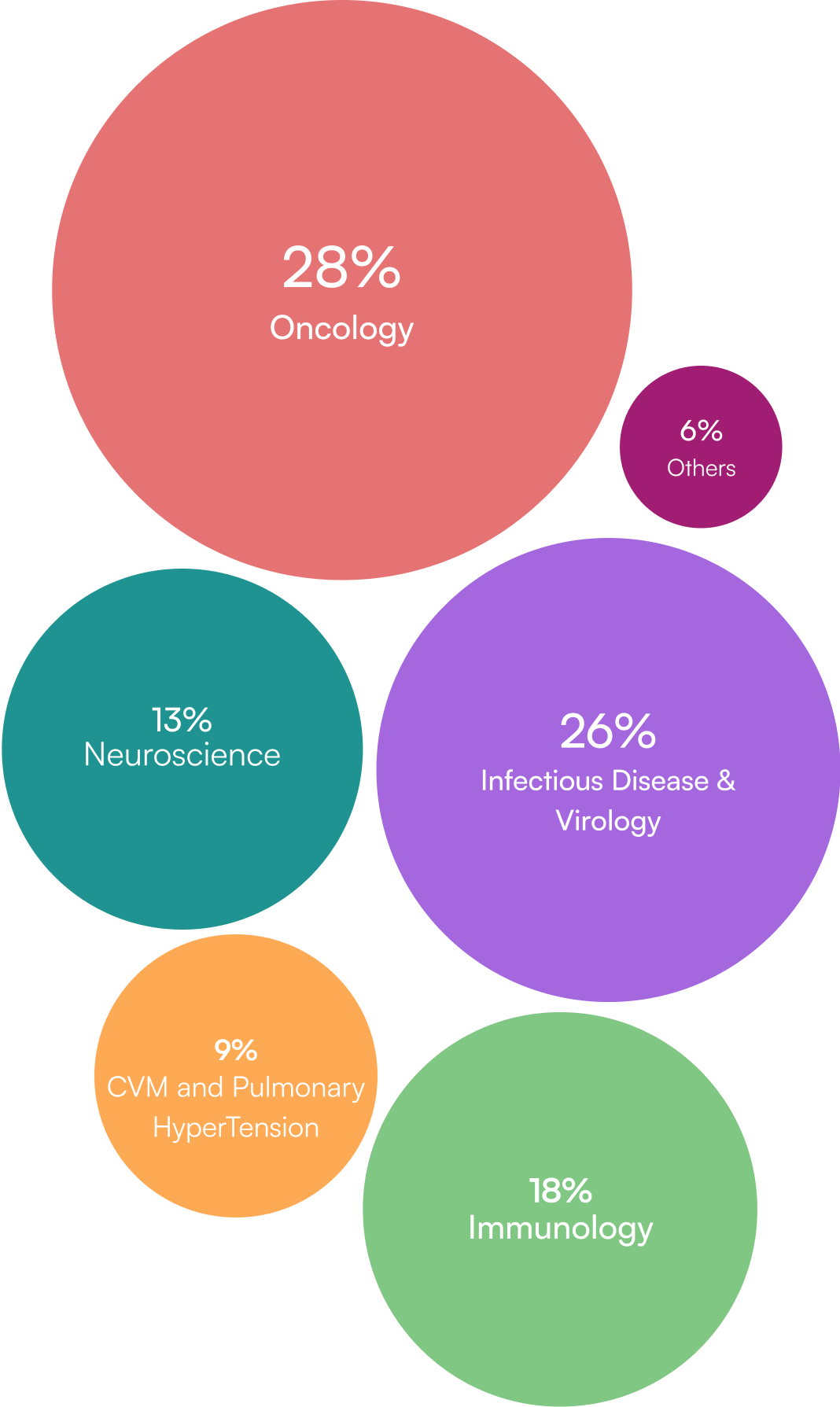
Global Pioneer in DSUR
Core Experts in Risk Management Plan
Innovation
Dedicated Experts Across All Major Therapeutic Areas
Automation